Home » Research Advances
Abstract
Engineered surfaces with reversibly switching interfacial properties, such as wettability and liquid repellency, are highly desirable in diverse application fields but are rare. We have developed a general concept to prepare metallic porous surfaces with exceptionally powerful wettability switch capabilities and liquid-repellent properties through an extremely simple one-step electrochemical deposition process. The wettability switch and manipulative liquid-repellent properties are enabled by orientation change of the dodecyl sulfate ions ionically bonded to the porous membranes during electrodeposition. The porous membrane with adjustable wettability enables it to trap different lubricants on demand within the pores to form liquid-infused porous surfaces with varied liquid-repellent properties. We have demonstrated the application of the (liquid-infused) porous membrane in encryption, controllable droplet transfer, and water harvesting. Moreover, the silver porous membrane can be coated onto a copper mesh, forming a smart antifouling liquid gate capable of allowing water or oil to pass through on request.
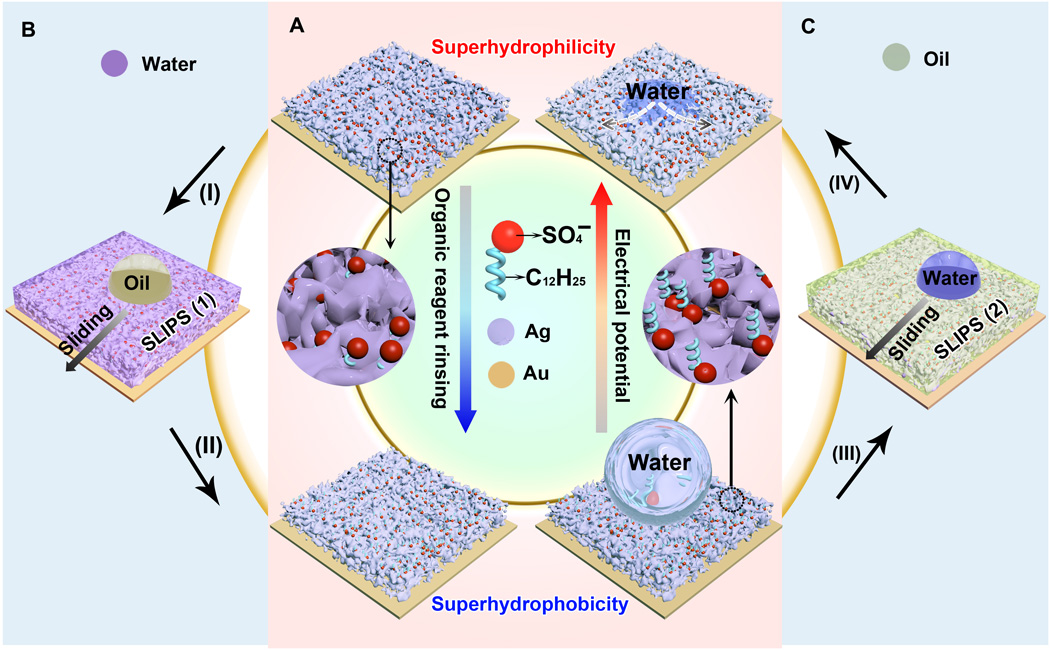
Fig. 1 Reversibly switching wettability and liquid repellency of the electrodeposited silver porous membranes.
(A) Reversible wettability transition from superhydrophilic to superhydrophobic, enabled by the orientation change of the dodecyl sulfate ions. (B) SLIPS 1 is formed by infusing water into the superhydrophilic porous membrane (process I), which can repel oil. After rinsing with ethanol (process II), SLIPS 1 turns superhydrophobic. (C) Oil is infused into the superhydrophobic porous membrane (process III), forming SLIPS 2. Water will be repelled by SLIPS 2. The lubricating oil will be released from SLIPS 2 under an electrical potential (process IV), giving rise to a superhydrophilic porous membrane.
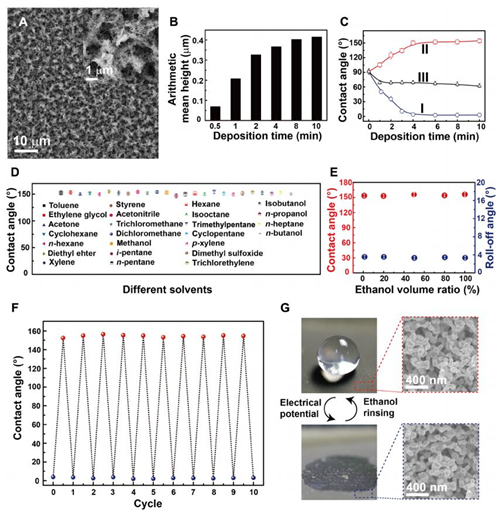
Fig. 2 Fabrication and characterization of the sliver porous membranes.
(A) Scanning electron microscope image of the electrodeposited silver porous membrane. Inset: Magnified image. (B) The arithmetical average roughness height of the silver membranes electrodeposited at 1.5 V for different times. (C) Curves I and II are the water contact angles on the as-prepared and the ethanol-treated silver porous membranes prepared at 1.5 V for different times, respectively. Curve III is the water contact angles on the ethanol-treated silver nanoparticle film electrodeposited in pure silver nitrate aqueous solutions. (D) The wettability transition from superhydrophilic to superhydrophobic can be completed by treatment with 25 commonly used organic reagents. (E) Contact angles and roll-off angles of the superhydrophilic silver porous membrane after being treated by a mixture of water and ethanol at different volume ratios. (F) The wettability transition from superhydrophilic to superhydrophobic and back to superhydrophilic for 10 cycles. (G) The morphology of the porous membrane is unchanged after 10 cycles of wettability transition. The error bars in (C) to (F) are obtained on the basis of at least five independent measurements
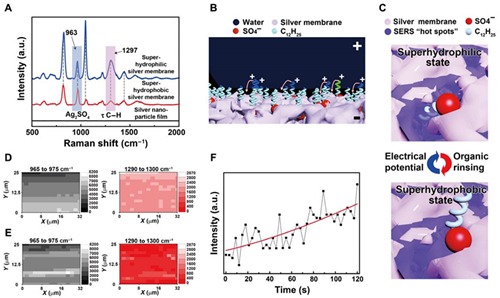
Fig. 3 Mechanism of the reversibly switching interfacial properties.
(A) SERS spectra of the silver porous membrane at superhydrophilic state and superhydrophobic state and the electrodeposited silver nanoparticle film in pure silver nitrate aqueous solutions. The SERS peaks located at 963 and 1297 cm−1 are assigned to silver sulfate and the torsional vibration mode (��) of C��H in the dodecyl chains, respectively. a.u., arbitrary units. (B) Schematic illustration of the orientation change of the dodecyl chains under an electrical potential. Positive charges will accumulate at the tips of the dodecyl chains contacting water, rotating them toward the negatively charged silver porous membrane. (C) Schematic illustration of the SERS intensity evolution of the dodecyl chains at different wetting states. At hydrophilic state, dodecyl chains are exposed to the SERS ��hot spots�� existing within the pores of the silver membrane, resulting in strong SERS signals. At hydrophobic state, the dodecyl chains are far away from the SERS hot spots, demonstrating weak SERS signals. (D and E) The SERS mapping results of silver sulfate and the dodecyl chains when the porous membrane is superhydrophilic and superhydrophobic, respectively. (F) The intensity evolution of the 1297-cm−1 SERS peak from dodecyl chains at a specific location as the electrical potential was applied to the superhydrophobic silver porous membrane (photo credit: Yue Liu, Zhejiang University).
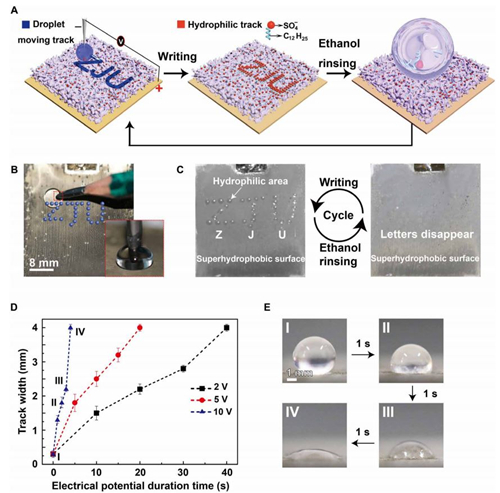
Fig. 4 Application in encryption.
(A) Schematic of the information encryption process. A water droplet is dragged by a pencil connected to the negative pole of a power supply to write letters ZJU on the superhydrophobic surface connected to the positive pole of the power supply. The track turns hydrophilic. When the porous membrane is immersed in water or exposed to water steam, the ZJU letters will appear. Ethanol rinsing can turn the hydrophilic track superhydrophobic, allowing for repeatable usage. (B) The setup for the encryption application. Inset: A pencil behaving as a cathode immersed in a droplet sitting on the superhydrophobic surface. (C) Repeatable usage of the silver porous membrane in encryption applications. (D) The track width as a function of the duration time at 2, 5, and 10 V. The error bars are obtained on the basis of five independent measurements. (E) Photographs of the track created at 10 V for different times (photo credit: Yue Liu, Zhejiang University).
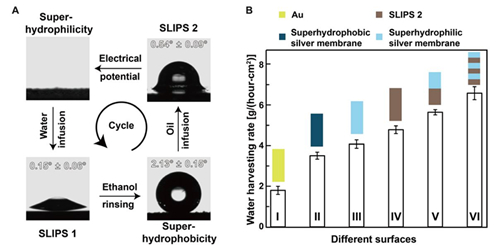
Fig. 5 Reversibly switching interfacial properties and applications in fog harvesting.
(A) Optical images of a water droplet sitting on the (liquid-infused) porous membranes at different states. Inset: The value of the contact angle hysteresis. The as-prepared superhydrophilic porous membrane can form SLIPS 1 by infusing water. SLIPS 1 turns superhydrophobic after ethanol rinsing. Oil can be infused into the superhydrophobic porous membrane, giving rise to the formation of SLIPS 2. SLIPS 2 can turn back to being superhydrophilic under an electrical potential treatment. This cycle can repeat for multiple times. (B) The water harvesting rate for different surfaces. Surface I, gold film; surface II, superhydrophobic silver porous membrane; surface III, superhydrophilic silver porous membrane; surface IV, SLIPS 2; surface V, the top half surface is superhydrophilic and the bottom half surface is SLIPS 2; surface VI, the surface composed of alternatively arranged superhydrophilic surface and SLIPS 2. The error bars are obtained on the basis of three independent measurements.
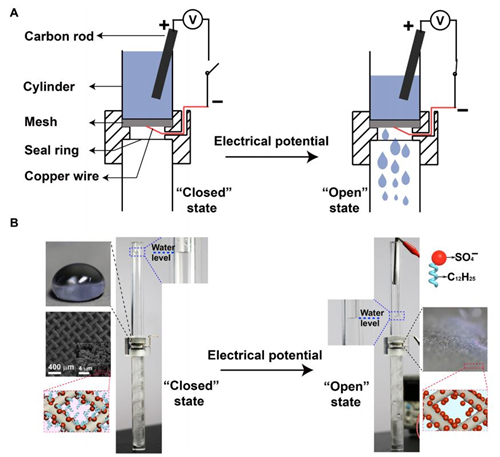
Fig. 6 Application as a smart liquid gate.
(A) Schematic of the setup of the smart liquid gate. (B) At the beginning, the mesh is at the ��closed�� state because the silver-coated copper mesh is superhydrophobic. Once the mesh is triggered by an electrical potential, it turns to the ��open�� state, and water starts to pass through the mesh. Inset: The image of a water droplet on and the orientation of the dodecyl sulfate ions on the silver-coated copper mesh at the closed and the open state, as well as the microstructure of the silver-coated copper mesh (photo credit: Yue Liu, Zhejiang University).
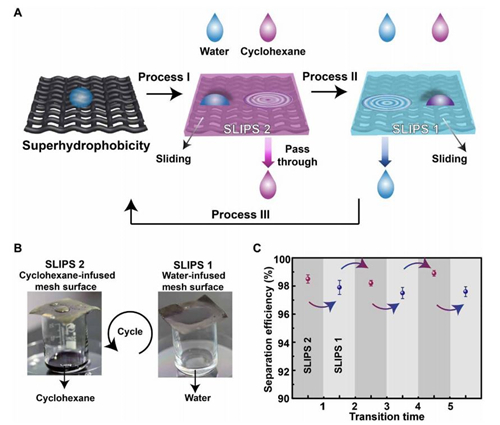
Fig. 7 Selective water/oil separation.
(A) Schematic illustration of the working mechanism of the water/oil separator. Process I: Infusing oil (here, cyclohexane) into the superhydrophobic porous membrane to form SLIPS 2. SLIPS 2 only allows cyclohexane to pass through; water will be left behind and slide away easily from SLIPS 2. Process II: When SLIPS 2 is subjected to an electric potential, the lubricating oil will be released from the porous surface. SLIPS 2 will turn superhydrophilic. The superhydrophilic porous membrane can trap water to form SLIPS 1. SLIPS 1 only allows water to pass through, while oil will be left behind and slide away from SLIPS 1. Process III: SLIPS 1 will turn superhydrophobic after being treated with ethanol. Processes I, II, and III form a cycle and can be repeated for desired times. (B) SLIPS 2 allows dyed cyclohexane to pass through, leaving behind water. SLIPS 1 allows water to penetrate, leaving behind dyed cyclohexane. (C) The oil/water separation efficiency of SLIPS 1 and SLIPS 2 at different transition times (photo credit: Yue Liu, Zhejiang University).
DISCUSSION
In summary, we have developed a general concept to engineer metallic coatings with switchable wettability and liquid-repellent properties via an extremely simple one-step electrodeposition process. The orientation change of the dodecyl sulfate ions ionically bonded to the electrodeposited porous metallic membrane triggered by organic reagent treatment, or an external electrical potential enables the wettability switch. The as-prepared silver porous membrane is superhydrophilic, with the dodecyl chains hidden inside the pores. The superhydrophilic porous membrane turns superhydrophobic when it is exposed to common organic reagents that can change the orientation of the dodecyl chains. The superhydrophobic silver porous membrane can return to being superhydrophilic when triggered by an external electrical potential. The wettability transition can be repeated more than 10 times. The switchable wettability allows the porous membrane to trap different lubricants, resulting in different SLIPS. The porous membrane can be changed from superhydrophilic, to water-infused SLIPS, to superhydrophobic, to oil-infused SLIPS, and further back to superhydrophilic, forming a cycle. We have demonstrated the application of the (liquid-infused) porous membrane with reversibly switching wettability in encryption, droplet transfer, sensing, and water harvesting fields. Furthermore, the silver membrane can be deposited onto a copper mesh, forming a smart liquid gate capable of allowing water to pass through when triggered by an electrical potential. The silver-coated copper mesh can allow water or oil to pass through on request by forming water- or oil-infused SLIPS, realizing highly efficient water/oil separation. We have demonstrated a general and extremely simple approach toward engineering metallic coatings with switchable wettability and liquid-repellent properties, which has promising applications in liquid and heat management�Crelated fields.
Science Advances 01 Nov 2019:
Vol. 5, no. 11, eaax0380
DOI: 10.1126/sciadv.aax0380
ShiKuang Yang Group��https://person.zju.edu.cn/shkyang
Website��https://advances.sciencemag.org/content/5/11/eaax0380/


